Tubular Glass Vials
Because sometimes you need the Perfect Type I Vial
Vision BioPharma offers a comprehensive range of ISO serum tubular glass vials in a corrugated plastic tray and lid design specifically for the 503A and 503B market to reduce the propensity of transportation and handling damage of smaller quantity requirements without compromising the highest quality Pharma+ standards of global USP, EP, and JP validation.
The vials are available in 2R to 50R (ml) sizes, in clear and amber glass of type I borosilicate (compliant to Pharma: Europe, Asia, USA). Drug Master Files Type I are available for all products on request.
Our Gx RTF serum tubular glass vials are of high quality and cover important characteristics regarding their usage for pharmaceutical applications:
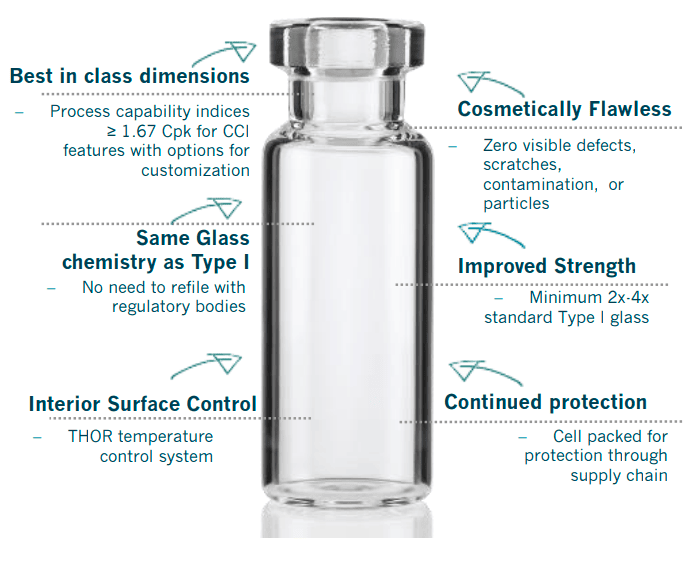
 Sizes ranging from 2 to 50 ml
Sizes ranging from 2 to 50 ml Clear and amber vials in glass
Clear and amber vials in glass Homogeneous walls for excellent cosmetic appearance
Homogeneous walls for excellent cosmetic appearance Tight tolerances and low dimensional variability
Tight tolerances and low dimensional variability Improved quality in labeling processes
Improved quality in labeling processes High thermal shock resistance
High thermal shock resistance Bottom concavity control for lyophilization vials
Bottom concavity control for lyophilization vials Ammonium sulfate treatments help to reduce alkali ion emission and the siliconization of the vials optimize the emptying characteristics
Ammonium sulfate treatments help to reduce alkali ion emission and the siliconization of the vials optimize the emptying characteristics
Quality assurance
 State-of the art production processes.
State-of the art production processes. In-line camera inspection systems to ensure high performance in filling lines and lyophilization processes.
In-line camera inspection systems to ensure high performance in filling lines and lyophilization processes. The production and packaging of Gx RTF serum tubular glass vials are performed under controlled environmental and cGMP conditions.
The production and packaging of Gx RTF serum tubular glass vials are performed under controlled environmental and cGMP conditions. Use of G3 vials inspection system to ensure the highest quality vials. During this inspection, all areas of the vials and finish, neck, shoulder, body, and hyper-bottom, are inspected. The system is based on a combination of five high-resolution matrices, in-line scan cameras, and a proprietary lighting system.
Use of G3 vials inspection system to ensure the highest quality vials. During this inspection, all areas of the vials and finish, neck, shoulder, body, and hyper-bottom, are inspected. The system is based on a combination of five high-resolution matrices, in-line scan cameras, and a proprietary lighting system. The custom software can recognize and classify defects.
The custom software can recognize and classify defects. In summary, Gx RTF vials production process is a precision handling system for repeatability.
In summary, Gx RTF vials production process is a precision handling system for repeatability.


 Vision BioPharma
Vision BioPharma  8007950850
8007950850